
TAP Psychopharmacology Bulletin - New Horizons / 2025 November
Prepared for clinicians and researchers working in the field of psychiatry.
Bulletin Subscription
Be the first to know when new issues are published! Apply now for free subscription.
Free • Cancel anytime you want
News
Brilaroxazine: A Critical Threshold for a Next-Generation Antipsychotic
Brilaroxazine, developed by Reviva Pharmaceuticals, has recently attracted significant attention as a promising antipsychotic agent for the treatment of schizophrenia. The molecule exhibits a broad pharmacological profile, acting on dopamine, serotonin, norepinephrine, and histamine receptors, thereby demonstrating therapeutic potential for both positive and negative symptoms. This multi-receptor targeting provides a pharmacodynamic advantage that distinguishes it from currently available antipsychotics. The recently published open-label extension (OLE) study has yielded important findings regarding the long-term efficacy and safety of brilaroxazine. Participants in the study demonstrated significant improvements in total Positive and Negative Syndrome Scale (PANSS) scores, along with notable reductions in depressive symptoms and enhanced overall functioning. These results suggest that the drug may target not only psychotic symptoms but also co-occurring affective manifestations.
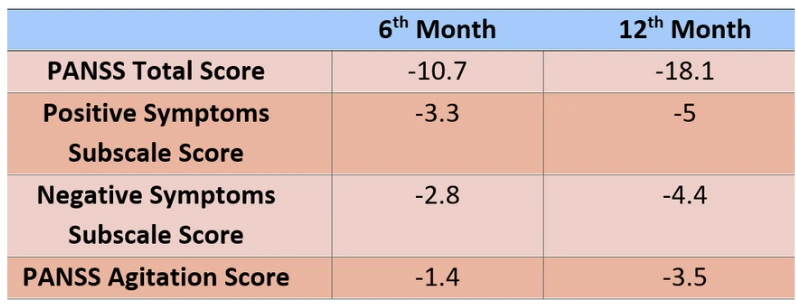
One of the most remarkable findings is Brilaroxazine’s favorable side-effect profile. Clinical trials have shown minimal association with weight gain, sedation, or extrapyramidal symptoms.
- Recent Developments in Bipolar Depression: An Interview with Prof. Mehmet Çağdaş Eker
- Current Debates on SSRIs in Pregnancy
- Glucagon-LIke PeptIde 1 (GLP-1)
- For Treatment Resistant Depression: Eskatemine
- Brexpiprazole Utilization in Adults and Children–Adolescents
This characteristic represents a key advantage for improving patient adherence in chronic conditions such as schizophrenia, which require long-term pharmacotherapy. Furthermore, no adverse effects have been observed on cardiac safety parameters, including QTc prolongation. Overall, Brilaroxazine presents a balanced profile in terms of both efficacy and tolerability. The drug’s clinical development has reached a critical milestone with the upcoming End-of-Phase 3 meeting with the FDA, which will serve as the primary determinant for its regulatory submission.
If the approval process proceeds successfully, Brilaroxazine may offer new therapeutic options not only for schizophrenia but also for other psychiatric disorders such as bipolar disorder, major depressive disorder, and attention-deficit/hyperactivity disorder (ADHD). Reviva Pharmaceuticals is planning additional hase trials to explore these expanded indications. To date, Brilaroxazine’s pharmacological characteristics and clinical evidence distinguish it as a truly next-generation antipsychotic candidate. Its low burden of side effects may increase its potential as an alternative option for treatment-resistant cases.
The forthcoming period will determine how this molecule will position itself within psychiatric practice. Upon FDA approval, Brilaroxazine is expected to make a significant impact in both clinical and academic psychiatry.
Dr. Gözde Çolak
DOI: 10.1007/asdere_55214_2023_416
Onfasprodil Shows Promise in Treatment-Resistant Depression
Treatment-resistant depression (TRD), defined as failure to respond to at least two adequate antidepressant trials, affects approximately one-third of individuals with major depressive disorder.
A multicenter phase 2 randomized, placebo-controlled study published in The Journal of Clinical Psychiatry investigated the efficacy of onfasprodil (MIJ821), a novel NMDA NR2B subunit antagonist.
The study enrolled 70 patients, who were randomly assigned to receive onfasprodil (at varying doses and frequencies), ketamine, or placebo for comparison. Results showed that onfasprodil produced a significant improvement in depressive symptoms within 24 hours, with the effect—particularly at the low dose of 0.16 mg/kg administered biweekly—lasting up to Week 6. Montgomery-Åsberg Depression Rating Scale (MADRS) scores improved by 5–8 points compared with placebo, a difference considered clinically meaningful. Compared with ketamine, onfasprodil demonstrated comparable efficacy and was associated with fewer dissociative side effects. The most commonly reported adverse events were dizziness, transient amnesia, and somnolence. Most were mild and resolved spontaneously within hours. Serious adverse events were rare and not directly related to the study drug.
The researchers emphasize that these results suggest onfasprodil could be a rapid-acting and well-tolerated treatment option for TRD. However, they note that the findings are limited by the small sample size and require confirmation in larger-scale trials. These early findings represent a potential new avenue of hope beyond current treatment options for treatment-resistant depression.
Dr. Gözde Çolak
DOI: 10.4088/JCP.23m15246
From the Editor...

Dear Colleagues, We are back again with the second issue of the Clinical Psychopharmacology Bulletin. In this issue, we have aimed to prepare content featuring new developments at the intersection of psychiatry and pharmacology. Even within just the last two months, there have been so many exciting advances that we had difficulty deciding which of them to share with you.
Although we frequently criticize the DSM system of the American Psychiatric Association for its shortcomings and flawed approaches, it continues to be widely used in clinical practice due to its practicality (even if we record diagnostic codes using the ICD system). At present, no alternative system seems poised to rival the DSM in either scientific or popular use in the short term. The NIMH’s (National Institute of Mental Health) proposal, the RDoC (Research Domain Criteria), has not gained broad application even in research settings due to its lack of practicality. Meanwhile, new proposals for the sixth version of the DSM have already started to emerge. The majority of these proposals focus on diagnostic fluidity and subtyping. In both of these areas, studies employing machine learning have been the driving force.
One of the most prominent disorders in the field of subtyping is major depressive disorder. Unlike earlier classifications based on phenomenology, studies are increasingly emphasizing biological parameters related to inflammation (IL-6, TNF, and CRP). The relationship between the immune system and depression has long attracted the attention of clinicians. While individuals experiencing acute infections may show transient depressive-like symptoms, patients with chronic autoimmune diseases display high rates of depression independent of their primary illness.
Among patients treated with INF-α, approximately 25–50% develop depression, while in experimental settings, animals with elevated immune parameters exhibit a pronounced functional deterioration of reward system activity, resembling a progressive blunting or ‘numbing’ of reward processing. Human studies, in turn, have revealed a negative association between CRP levels and reward system activity. Large-scale studies have shown that about 25–30% of patients with depression present with increased immune parameters (CRP > 3 mg/L – low-grade inflammation). In this subgroup, responses to serotonergic antidepressants are poorer compared to other patients; however, better outcomes are achieved when noradrenergic and dopaminergic agents are added to treatment. In the same subgroup, anhedonia and neurovegetative symptoms appear more frequently. High BMI (via lipid peroxidation) and low levels of physical activity are thought to contribute to the inflammatory processes. It is also noteworthy that patients with a history of childhood trauma exhibit higher levels of these markers and that elevated markers are associated with greater treatment resistance.
The use of anti-inflammatory and anti-cytokine agents has been shown to reduce depressive symptoms in patients with autoimmune diseases, independent of the primary illness. In patients with primary depression, results are less consistent; however, positive effects have been reported in those with CRP > 3 mg/L.
In conclusion, one quarter of patients with depression—and an even higher proportion of those with treatment-resistant depression—display an immune profile distinct from other patients. This situation, pointing to a separate pathophysiological mechanism, suggests that in the next version of the DSM, these immune markers could be incorporated as a new specifier within depressive subtypes. Such an approach would be an important step toward the development of personalized treatments.
Best regards,

- Prof. Dr. M. Kemal SAYAR
- Prof. Dr. Mesut ÇETİN
- Prof. Dr. Feyza ARICIOĞLU
- Prof. Dr. Ali Saffet Gönül
- M.D. Gözde Çolak
- Yiğit Erdoğan
psychopharmaupdate@gmail.com
Its content is independent of the pharmaceutical industry.
RECENT DEVELOPMENTS IN BIPOLAR DEPRESSION:
AN INTERVIEW WITH PROF. MEHMET ÇAĞDAŞ EKER
Dr. Özgür Değirmenci

In Bipolar Affective Disorder, the majority of the disease burden arises from depressive episodes, which account for approximately two-thirds of the illness duration. Moreover, chronic and subthreshold depressive symptoms are frequently observed even outside acute episodes. The treatment of bipolar depression remains challenging, and current clinical guidelines provide more limited recommendations compared with those for manic episodes and maintenance therapy. In view of these challenges we had the opportunity to interview Prof. Dr. Mehmet Çağdaş Eker and to obtain his views.
ÖD: We often see that many patients with bipolar depression were initially followed under diagnoses of unipolar depression or treatment-resistant depression. In the differential diagnosis process, which clinical clues or historical details can be decisive in clarifying the diagnosis?
If a patient presenting in the depressive phase is not carefully evaluated for a history of bipolarity, there is a risk of misdiagnosis as unipolar (major) depression.
MÇE: Bipolar Disorder is generally an illness dominated by depression. For this reason, patients most often present to physicians during depressive periods. Moreover, our patients do not describe episodes of mania because they do not consider them to be an illness. If I were to select three features that would raise suspicion for bipolar disorder during a depressive episode, first would be an increased need for sleep. A history of depression or another psychiatric disorder after childbirth, and a family history of bipolar disorder, would be the other features.
ÖD: According to DSM-5, depression with mixed features is defined within both unipolar and bipolar frameworks. In your view, how well does the definition of mixed symptoms in unipolar depression match real-world clinical populations?
MÇE: I believe that mixed-feature depression also falls within the bipolar spectrum and should be treated like bipolar disorder. However, the diagnostic criteria for mania required in defining mixed-feature depression do not include irritability, psychomotor agitation, or distractibility. This creates difficulties in diagnosing mixed-feature depression and in recognizing the broader bipolar spectrum.

ÖD: Many treatment guidelines recommend agents such as lithium, lamotrigine, quetiapine, and lurasidone as first-line options for bipolar depression. In your clinical practice, what factors determine your choice among them?
MÇE: When treating the acute phase of bipolar disorder, we must plan with long-term medication use in mind. While we expect treatment to alleviate depression rapidly, it must also be sustainable in terms of side effects and efficacy. Quetiapine, lurasidone, and lithium stand out for their rapid effectiveness, whereas lamotrigine is preferred because its side effects are rare and it improves adherence. In choosing a drug, not only the severity of depression and clinical features but also clinical history will be decisive. For example, lithium may be prioritized for a patient with suicidal thoughts or prior suicide attempts, while lamotrigine may be a first choice for a woman with a mild depressive episode.
ÖD: Lithium has long been recognized as an effective treatment in bipolar disorder, both during depressive episodes and in maintenance therapy. In light of current clinical evidence, how would you evaluate lithium’s effectiveness in bipolar depression?
MÇE: Lithium is an “orphan” drug. Conducting drug research costs millions of dollars, and because lithium is not supported by the pharmaceutical industry, it is not easy to find studies on lithium. On the other hand, both older studies and clinical observation show that lithium works for many patients. Lithium begins to show its effect within 2–3 weeks after the serum level reaches the therapeutic range. Its rapid effect distinguishes it from other drugs. However, challenges in monitoring serum levels and issues with adherence create risks and limit its use. If same-day lithium level testing is available and the patient’s clinical profile is suitable, I can say that using lithium can be lifesaving.
ÖD: With lurasidone recently introduced in our country, its role in the treatment of bipolar depression has become a subject of increasing discussion. In light of current evidence, how effective do you consider lurasidone to be for bipolar depression, and how do you approach its use in your own clinical practice?
MÇE: Although lurasidone is approved for bipolar depression, it has not yet been included in reimbursement, so it has not found widespread use. With its rapid onset and flexible dosing, I expect lurasidone to fill an important gap. I have also had the opportunity to observe effects on obsessions and anxiety in patients using lurasidone. On the other hand, I encountered akathisia and dystonia more frequently than expected. In the dystonia cases I saw, tongue enlargement was particularly notable, but it resolved quickly with biperiden.
ÖD: The olanzapine–fluoxetine combination (OFC) is highlighted as an important option, especially for treatment-resistant cases and bipolar depression, and its effectiveness has been demonstrated in many studies. What is your view on its place in bipolar depression?
MÇE: I do not think the olanzapine–fluoxetine combination differs from combining olanzapine with another antidepressant. I suspect this combination emerged because both drugs are produced by the same company. Moreover, in comparative studies with OFC, there are either no data or very limited data for:
(1) comparisons of olanzapine combined with other antidepressants;
(2) comparisons with lithium plus antidepressant combinations;
(3) comparisons of other antipsychotics with fluoxetine or other antidepressants.
In this respect, there is insufficient scientific basis to favor OFC specifically; however, I may be able to state a more definitive view as clinical experience accumulates.
ÖD: In the treatment of bipolar depression, particularly in treatment-resistant cases, antidepressants are often used. Guidelines consider this approach reasonable, provided it is applied cautiously. Could you describe your clinical practice in this regard? In addition, are there any antidepressants regarded as safer with respect to the risk of a manic switch?
MÇE: The main limitation in choosing an antidepressant is their insufficient efficacy in bipolar depression and the potential for polypharmacy. The risk of a manic switch generally remains below 20%. Systematic reviews and meta-analyses indicate a tendency for agents with anticholinergic properties and broad receptor profiles to carry greater risk.
Prior to initiating an antidepressant in bipolar depression, I recommend carefully evaluating how previous manic episodes began, whether a recent manic episode has occurred, and whether mixed symptoms are present.
ÖD: In cases where bipolar depression is particularly severe, psychotic, catatonic, and/or treatment-resistant, electroconvulsive therapy (ECT) is an important option. Based on your clinical experience, which patient profiles benefit from ECT, and what criteria do you consider when deciding on this method? What are your views on seizure duration and session frequency?
MÇE: Unfortunately, there is no definitive way to know which patients will respond to ECT, but there are some clues. For example, there is evidence that it is more effective in melancholic depressions or in depressions where anhedonia is prominent. We also see it work in depressions with catatonia or severe psychomotor retardation. Seizure duration needs to be monitored by EEG, though this may not always be possible due to technical issues. A seizure duration of at least 20 seconds is required by EEG; if it is under 15 seconds, repeating the application within the same session is recommended. For severe patients, sessions can start three times per week and then be reduced to twice weekly. In severe malignant catatonia, the first three sessions are recommended on three consecutive days.
ÖD: Ketamine and esketamine have attracted attention in recent years with their rapid antidepressant effects. What are your thoughts on their role in bipolar depression?
MÇE: Ketamine and esketamine stand out with rapid action and anti-suicidal effects. However, in many resistant cases they are considered just before ECT. This means they are tried in chronic, difficult-to-treat patients.
In such challenging cases, as with many treatments, it is not easy to obtain a response. Even to ketamine and esketamine. I believe trying these treatments earlier in the course may increase the likelihood of success.
ÖD: Attention-Deficit/Hyperactivity Disorder (ADHD) is frequently comorbid with bipolar disorder. Under what clinical circumstances do you find it appropriate to initiate treatment for ADHD?
MÇE: Comorbid ADHD is common in patients with bipolar disorder and often presents severely enough to require treatment. If the patient does not experience frequent manic episodes, does not present with psychotic symptoms, and demonstrates adequate treatment adherence, I believe that severe comorbid ADHD should be treated, as it contributes to additional problems such as disruption of biological rhythms, substance use, and adherence difficulties. However, the patient must meet certain conditions. First, strict adherence to sleep hygiene is required; the patient should not stay up past 11 p.m.Since caffeine misuse is a significant problem accompanying ADHD, I limit patients to two coffee equivalents per day and ask them to stop caffeine intake after 12 noon. Regular exercise is the most recommended lifestyle adjustment for both ADHD and bipolar disorder, so I insist on it. Provided these conditions are met, I can initiate methylphenidate or atomoxetine therapy and monitor closely.
CURRENT DEBATES ON SSRIs IN PREGNANCY:
FDA PANEL AND EXPERT OPINIONS
Dr. İrem Erbaş

On July 21, 2025, the U.S. Food and Drug Administration (FDA) convened an expert panel of renowned clinicians, researchers, and health policy makers to evaluate possible labeling changes regarding the use of selective serotonin reuptake inhibitors (SSRIs) during pregnancy. The panel addressed critical issues such as the role of the serotonin system in fetal development, the potential short- and long-term effects of SSRI exposure, and how to establish a risk–benefit balance in treating depression during pregnancy. The purpose of the panel was to review scientific evidence and to discuss the scope of current warnings in a way that would inform both clinicians and the public. However, many experts in perinatal psychiatry and obstetrics expressed concerns afterward, stating that while the panel emphasized risks, it did not sufficiently take into account the serious consequences of untreated depression and risked creating the impression of a one-sided presentation that could undermine scientific balance.
Key Points Highlighted in the Panel
Dr. Marty Makary, professor of surgery at Johns Hopkins University, emphasized that the risks of SSRI use in pregnancy should not be evaluated solely in relation to the medication but rather within a broader context that includes factors such as healthy lifestyle, community support, and natural light.
Dr. Anick Bérard, a pharmacoepidemiologist at the University of Montréal, pointed out that the use of paroxetine and fluoxetine has been associated with some adverse pregnancy outcomes, but argued that these risks must be assessed in absolute terms.
Dr. Jay Gingrich, a developmental neurobiologist from Columbia University, stated that SSRI exposure may alter emotional processing regions in the infant brain and therefore argued that warnings related to fetal brain development should be included on drug labels. Dr. Adam Urato, chief of maternal–fetal medicine at MetroWest Medical Center, noted that SSRI use could increase the risk of neurological problems emerging during adolescence in female children, calling for better public awareness.
Dr. David Healy, known for his critical work in psychopharmacology, argued that evidence for the effectiveness of SSRIs is weak even in severe depression and that discontinuation can lead to prolonged withdrawal syndromes.
Clinical psychologist Dr. Roger McFillin highlighted the limited efficacy and significant side effects of SSRIs, stressing the importance of lifestyle interventions and therapeutic methods.
Dr. Josef Witt-Doerring, a psychiatrist formerly with the FDA and founder of TaperClinic, noted that SSRI withdrawal symptoms are underestimated and that there are serious gaps in informing patients. Dr. Kay Roussos-Ross, who works in both obstetrics and psychiatry at the University of Florida, emphasized that untreated depression can lead to serious complications such as preeclampsia and preterm birth during pregnancy, and therefore SSRIs may be life-saving for some patients. Finally, Dr. Joanna Moncrieff from University College London argued that antidepressants have limited efficacy, that depression often resolves spontaneously, and that the risk–benefit balance must be carefully evaluated for each patient.
Reactions of the Experts After the Panel
The American College of Obstetricians and Gynecologists (ACOG) and the American Psychological Association (APA) stated that the panel highlighted risks while failing to sufficiently emphasize the well-documented harms of untreated depression. The Massachusetts General Hospital Center for Women’s Mental Health criticized the panel for having too few experts with direct experience in reproductive psychiatry. Perinatal psychiatrist Dr. Sunny Patel noted that the panel was presented in a way that could foster fear and misinformation among the public.
The National Center for Reproductive Psychiatry (NCRP) stated that current evidence does not show a direct causal relationship between prenatal SSRI exposure and congenital anomalies or autism spectrum disorders.
Conclusion
The use of SSRIs during pregnancy continues to be one of the controversial issues in modern psychiatry. The FDA panel held on July 21, 2025, brought the risk–benefit balance at the heart of this debate back into focus, highlighting both the critical role of the serotonin system in fetal development and the importance of contradictory findings regarding SSRI exposure. The views presented underscored the potential congenital and neurodevelopmental risks of SSRIs while also pointing out that the maternal and perinatal consequences of untreated depression cannot be ignored.
From a clinical perspective, SSRIs may be life-saving in some cases, whereas non-pharmacological approaches may be safer first-line options for mild or moderate depression. This underscores the need for treatment planning to be based on individual risk profiles, illness severity, stage of pregnancy,
The panel discussions underscored both the potential congenital and neurodevelopmental risks of SSRIs and the importance of not neglecting the maternal and perinatal consequences of untreated depression.
and patient preferences. From an ethical standpoint, a delicate balance must be established between patient autonomy, nonmaleficence, and beneficence. Patients’ access to accurate, transparent, and balanced information is an essential component of the informed consent process. Criticism following the panel mainly focused on the insufficient representation of obstetrics and perinatal psychiatry experts and the one-sided emphasis on risks. The reactions of organizations such as ACOG, APA, and the MGH Center for Women’s Mental Health highlight the importance of subject-matter expertise and interdisciplinary perspectives in scientific debates. In this respect, creating more balanced and inclusive panel compositions in future evaluations is of great importance.
On the research front, significant gaps remain. Findings on the long-term neurodevelopmental outcomes of SSRI exposure are inconsistent, and larger, longitudinal studies are needed. Similarly, more controlled research is required to better elucidate the maternal and perinatal risks of untreated depression, which would facilitate clinical decision-making. In conclusion, it is clear that a one-size-fits-all approach is not possible for SSRI use during pregnancy. The decision-making process should be personalized in light of existing scientific evidence and carried out through transparent, interdisciplinary collaboration among clinicians, researchers, and policymakers. In this context, developing a risk communication strategy that protects both maternal health and fetal development while also fostering public trust should be one of the main goals moving forward.
GLUCAGON-LIKE PEPTIDE 1 (GLP-1):
IT EMERGING ROLE IN ADDICTION
Dr. İbrahim Sungur

What is GLP-1?
Glucagon-like peptide 1 (GLP-1) is an incretin hormone secreted throughout the gastrointestinal tract. It plays a vital role in glucose metabolism, satiety, and energy balance. Beyond its classical metabolic functions, increasing evidence highlights GLP-1’s central nervous system (CNS) activity, suggesting it may represent a promising target in treating addiction. Although the majority of the GLP-1 receptors (GLP-1Rs) are expressed in the gastrıintestinal system, various human tissues, including muscle, adipose tissue, bones, and numerous organs such as brain, express GLP-1Rs. These receptors mediate several physiological processes, including glucose homeostasis, gastric motility, lipid metabolism, and cardiovascular and neurologic functions.
Current Therapeutic Uses of GLP-1 Receptor Agonists (GLP-1RAs)
The therapeutic potential of GLP-1 receptor agonists (GLP-1RAs) has been well established in type 2 diabetes, cardiovascular disease, and obesity. Currently approved GLP-1RAs include albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, and semaglutide. Notably, newer agents like semaglutide have gained attention due to their pronounced effects on weight reduction, even among individuals without diabetes.
- DOI: 10.1210/en.2013-1934
- DOI: 10.3389/fendo.2021.721135
- DOI: 10.1016/j.drudis.2016.01.013
- DOI: 10.1186/s12974-019-1638-6
- DOI: 10.1210/en.2011-1443
- DOI: 10.3389/fphar.2023.1063033
- DOI: 10.1523/JNEUROSCI.3262-11.2011
- DOI: 10.1111/dom.16453
- DOI: 10.1016/j.physbeh.2024.114565
- DOI: 10.1016/j.physbeh.2019.03.026
- DOI: 10.1038/s41386-018-0010-3
The Role of GLP-1in Brain
Importantly, GLP-1 and its analogs can cross the blood-brain barrier and are also endogenously synthesized in specific brain regions, including the hypothalamic nuclei, nucleus tractus solitarius (NTS), and caudal brainstem.
GLP-1Rs are present in several key brain regions implicated in reward and motivation circuits, such as the caudate, putamen, globus pallidus, hypothalamus, amygdala, hippocampus, and ventral tegmental area (VTA).
In the central nervous system, GLP-1 has been shown to influence mitochondrial function, synaptic plasticity, and neuroinflammatory pathways, thereby exerting neuroprotective effects. These mechanisms have prompted investigation into GLP-1RAs for neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease.
GLP-1 and Addiction
Crucially, recent preclinical and clinical evidence suggests that GLP-1 also modulates the mesolimbic reward system, which is central to the neurobiology of addiction. GLP-1Rs in the VTA and nucleus accumbens influence dopamine-mediated behaviors associated with reward, craving, and reinforcement. Activation of these receptors has been found to attenuate behaviors induced by addictive substances, including alcohol, nicotine, opioids, cocaine, and amphetamines. Animal studies have demonstrated that GLP-1RA administration can reduce alcohol intake, blunt drug-seeking behaviors, and alleviate withdrawal symptoms. These effects appear to stem from both hypothalamic appetite-regulating circuits and direct projections from NTS GLP-1 neurons to mesolimbic regions. Taken together, the accumulating evidence positions GLP-1R agonists as potential therapeutic agents in the management of substance use disorders.
Alcohol:
Multiple large-scale observational studies found that using semaglutide or liraglutide had a significantly lower risk of hospitalization for alcohol-related issues compared to non-users. Semaglutide was associated with a 36% reduction, and liraglutide with a 28% reduction in risk.
Opioids:
Preclinical studies indicate GLP-1RAs reduce drug-seeking behavior and relapse in opioid use disorder.
Nicotine:
Early data suggest GLP-1RAs reduce nicotine self-administration, cravings, and withdrawal-related weight gain. They may also help mitigate post-cessation weight gain, a common barrier to quitting smoking.
Cocaine:
Preclinical studies show that GLP-1RAs reduce cocaine-seeking behaviors and may lower the risk of relapse by attenuating the reward response to cocaine.
Although GLP-1 receptor agonists show promise as potential treatments for various substance addictions, most robust data comes from animal models and observational human studies. Randomized controlled trials in humans are limited but ongoing. The preclinical and observational data are encouraging; more rigorous clinical trials are needed to confirm efficacy and safety in broader populations.
FOR TREATMENT RESISTANT DEPRESSION:
ESKETAMINE
Dr. Rahnuma Beheshti & Dr. Kaan Keskin

The Global Burden and Treatment Challenges of Depression
According to the World Health Organization, the global prevalence of depression in 2015 was reported to be 4.4%. In other words, approximately one in every 20 people worldwide is diagnosed with depression at some point in their life. Although psychotherapies and selective serotonin reuptake inhibitors (SSRIs) are available as first-line treatments for patients, the limitations of conventional methods used to manage depression pose significant challenges. SSRIs typically require about two to six weeks to become effective, and early side effects may paradoxically lead to feelings of agitation, anxiety, and apathy. Despite SSRI/SNRI treatment, the average remission rate is around 30–40%. Moreover, nearly one-third of patients with depression are treatment-resistant, meaning they do not respond to two different treatment strategies. However, new developments offer a promising outlook; one of these is the use of esketamine, a novel NMDA receptor antagonist, for individuals experiencing treatment-resistant depression.
Pharmacological Properties and Clinical Effects of Esketamine
Esketamine is the S-enantiomer of the racemic mixture of ketamine. In patients with depression, dysfunctional and overactive N-methyl-D-aspartate (NMDA) receptors may lead to reduced neuroplasticity. Ketamine works by blocking these receptors, which enhances glutamate release and signal transmission, thereby resulting in improvements in mood and behavior.Esketamine (S-ketamine) is twice as potent as ketamine and has a higher affinity for NMDA receptors, providing a much stronger and faster analgesic effect.
Findings indicate that the mean Montgomery–Åsberg Depression Rating Scale (MADRS) score did not decrease following esketamine administration; however, patients were able to sustain lower MADRS scores throughout the study.
Safety, Side Effects and Clinical Application
A recent study published in the International Journal of Neuropharmacology reported that esketamine monotherapy was statistically effective compared to a placebo-controlled group. Positive changes were observed within 24 hours after the first dose and persisted for up to one month throughout the treatment period. In the study’s long-term safety and efficacy extension focusing on prolonged use, researchers found no evidence of cognitive decline associated with continued administration. The response was relatively maintained over time, with only 5.3% of participants withdrawing from the study due to esketamine being reported as ineffective. In some participants, symptom scores continued to improve progressively. Nausea, dissociation, and dizziness are among the most commonly reported side effects by participants using esketamine.
Increases in blood pressure peak approximately 40 minutes after esketamine administration and persist for around four hours. Esketamine, at all currently recommended doses, causes elevations in systolic and/or diastolic blood pressure. Real-world studies
have reported rates of elevated blood pressure ranging from 10.0% to 14.0%. A transient psychotic episode associated with intranasal esketamine has been reported in a case study. In January 2025, Spravato, the patented nasal spray formulation of esketamine, was approved by the U.S. Food and Drug Administration (FDA) as a monotherapy for treatment-resistant depression. This approval has paved the way for broader access to and use of this medication, which has demonstrated significant clinical potential.
- DOI: 10.1176/appi.ajp.20170321
- DOI: 10.1176/ajp.2006.163.11.1905
- DOI: 10.1016/j.jad.2008.10.014
- DOI: 10.3390/biomedicines12102283
- DOI: 10.1001/jamapsychiatry.2025.1317
- DOI: 10.1093/ijnp/pyaf027
- DOI: 10.1093/ifhsp/asfepy237727
- DOI: 10.1016/j.jad.2022.09.043
- DOI: 10.1080/13651501.2022.2030757
- DOI: 10.1016/j.psycr.2024.100236
BREXPIPRAZOLE UTILIZATION IN ADULTS AND CHILDREN–ADOLESCENTS:
CURRENT PERSPECTIVES
Dr. İpek Aksakal

Introduction
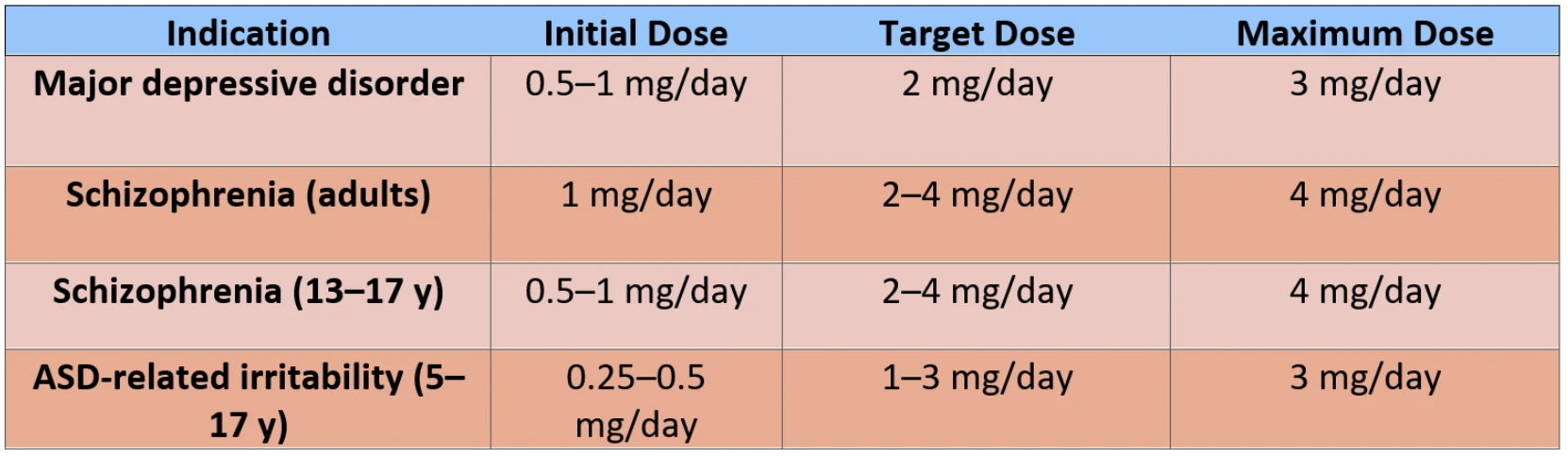
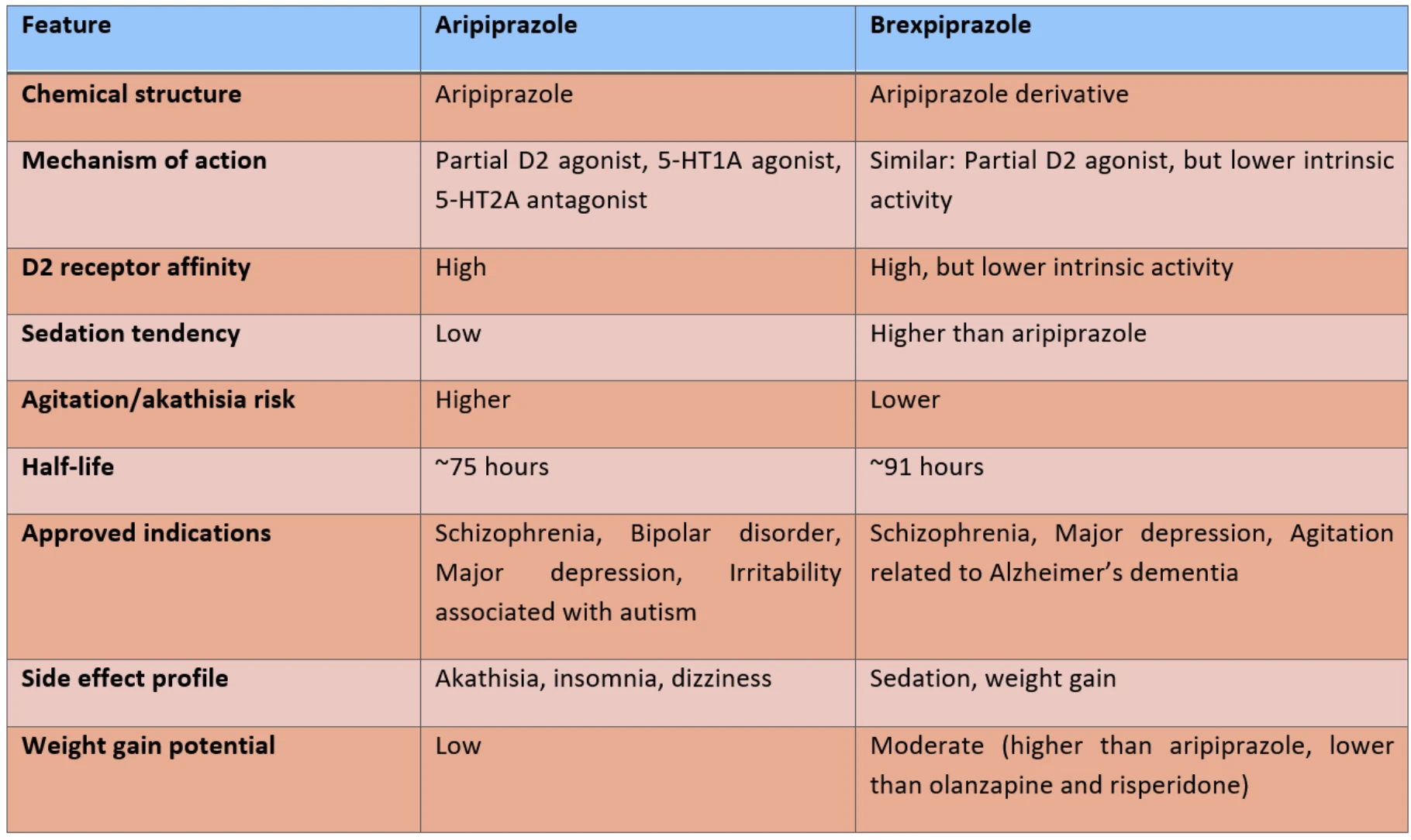
Brexpiprazole is an atypical antipsychotic indicated for the treatment of schizophrenia, major depressive disorder, agitation associated with Alzheimer’s dementia, other psychotic disorders, post-traumatic stress disorder (PTSD), and behavioral problems in children and adolescents. In adults, brexpiprazole is approved as an adjunctive treatment for schizophrenia and major depressive disorder, offering pharmacodynamic similarities to aripiprazole but with a different dosing range and side effect profile. Although not yet approved for pediatric use, several clinical studies have been conducted.
Mechanism of Action
Brexpiprazole acts primarily as a serotonin 5-HT2A receptor antagonist, and as a partial agonist at dopamine D2 and serotonin 5-HT1A receptors. It also blocks α1- and α2-adrenergic receptors, which contributes to sedative and antidepressant effects and may alleviate PTSD-related symptoms. This pharmacological profile not only alleviates psychotic symptoms but also helps manage depressive and anxiety symptoms. Compared with aripiprazole, brexpiprazole exhibits lower intrinsic activity at D₂ receptors, which contributes to a reduced risk of side effects.
Use in Children and Adolescents
Although not formally approved, brexpiprazole has been used in clinical practice with children and adolescents. Its potential applications have been investigated in schizophrenia, bipolar disorder, depressive disorders, and in irritability associated with autism spectrum disorder (ASD). However, data on long-term safety and efficacy remain limited. A multicenter, randomized, double-blind phase 3 study in adolescents aged 13–17 years demonstrated that brexpiprazole significantly reduced schizophrenia symptoms compared to placebo, with favorable tolerability. Phase 3 trials in children and adolescents aged 5–17 years with ASD-related irritability reported efficacy and safety, suggesting brexpiprazole as a potential treatment option. In an open-label study evaluating long-term safety in adolescents, the mean weight gain after 52 weeks was 2.1 kg. Clinically relevant weight gain (≥ 7% increase) was observed in 18.6% of participants, while no significant deterioration in metabolic parameters was reported.
5-HT7 receptor antagonism plays a role in reducing cognitive and negative symptoms, while partial agonism at dopamine D3 receptors supports antidepressant effects and improvement of negative symptoms.
These findings suggest that brexpiprazole is well tolerated in terms of weight gain and metabolic effects during long-term pediatric use, with its metabolic side effects potentially being milder compared with risperidone and olanzapine.
Adverse Effects
The most common adverse effects are sedation, orthostatic hypotension, weight gain, and akathisia. Although the incidence of akathisia increases with higher doses, it is reported to occur less frequently compared with aripiprazole. In open-label studies, the mean annual weight gain in patients was reported as 2.1 kg. With respect to prolactin levels, mild elevations were observed in approximately 10% of cases, whereas clinically significant prolactin increases were reported in about 1%.
Before Initiating Brexpiprazole and During Follow Up
Prior to initiating brexpiprazole treatment, and in line with general recommendations for atypical antipsychotics (AAPs), patients should be evaluated for body mass index (BMI), comorbid medical history, waist circumference, blood pressure (BP), fasting blood glucose (FBG), and lipid profile.
Dosage and Administration
Brexpiprazole’s long elimination half-life of 91 hours allows once-daily dosing, improving adherence. Food intake does not affect absorption. Peak plasma levels are reached approximately 4 hours after dosing, with steady-state concentrations achieved by days 10–12.
Oral bioavailability of brexpiprazole is high (95%), with approximately 99% binding to plasma proteins. Brexpiprazole is primarily metabolized via the CYP3A4 and CYP2D6 enzymes. Clinically significant drug interactions are rare; however, when co-administered with a CYP3A4 inhibitor, the dose should be reduced by half, while concomitant use with a CYP3A4 inducer requires doubling the dose (up to a maximum of 12 mg/day), preferably administered in divided doses.
What Can Be Done If Brexpiprazole Treatment Is Insufficent?
In cases where brexpiprazole treatment is insufficient, switching to another atypical antipsychotic or considering combination therapy may be appropriate. The use of other atypical antipsychotics such as risperidone, olanzapine, and quetiapine is particularly common. Increasing the dose beyond established therapeutic limits has not proven effective and is therefore not recommended. As an adjunctive strategy, augmentation with agents such as valproate, lamotrigine, lithium, or benzodiazepines may also be considered.
Comparison with Aripiprazole
Brexpiprazole shows higher affinity for dopamine D1, D2, and D4 receptors, but lower affinity for D3 receptors compared with aripiprazole. It also exerts stronger antagonism at 5-HT2A receptors. These properties are associated with a lower risk of akathisia and insomnia, and a less activating profile. Its lower intrinsic activity may further reduce activation-related side effects. Stronger 5-HT1A partial agonism may explain more prominent antidepressant and anxiolytic effects.
Discussion and Conclusion
Brexpiprazole represents an important treatment alternative in adults owing to its efficacy and generally favorable tolerability. Its side-effect profile and long half-life may provide particular advantages in treatment-resistant cases. Although not yet approved for use in children and adolescents, brexpiprazole has been reported to have a side-effect profile similar to that observed in adults, with lower rates of sedation and metabolic adverse effects. When used as an adjunct to antidepressant therapy, brexpiprazole has demonstrated potential benefits in improving mood, anxiety, and agitation symptoms. Nevertheless, larger and more rigorously designed studies are required to determine the generalizability of these findings.
- DOI: 10.1111/ijcp.12714
- DOI: 10.1038/s41537-021-00171-2
- DOI: 10.4088/JCP.24m15577
- DOI: 10.4088/JCP.22f14593
- DOI: 10.1001/jamaneurol.2023.3810
- DOI: 10.2147/TCRM.S94060
- DOI: 10.1097/JCP.00000000000979
- DOI: 10.1002/jcph.1946
- DOI: 10.1016/S2215-0366(25)00043-4
- DOI: 10.1016/10.1089/cap.2024.0118
- DOI: 10.1016/10.1080/00484.2021.898
- DOI: 10.1016/10.1016/j.jagp.2019.09.009
- DOI: 10.1016/10.1093/ijnp/pyw076
- DOI: 10.1016/10.1002/npr2.12180
- DOI: 10.1016/10.1124/jpet.114.213793
Number Need to Treat (NNT)
Prof.Dr. Ali Saffet Gönül
The Number Needed to Treat (NNT) is an important metric used in clinical research and evidence-based medicine to evaluate treatment efficacy. It represents the average number of patients who need to receive an intervention for one additional patient to benefit, compared with a control group (typically placebo or standard treatment). In simpler terms, it indicates how many patients must be treated for one person to experience a beneficial effect. Mathematically, it is the inverse of the Absolute Risk Reduction (ARR):
ARR=CER* - EER**
*CER (Control Event Rate): The incidence of the event in the control group (the untreated or placebo group)
**EER (Experimental Event Rate): The incidence of the event in the experiment group (the group receiving the drug or treatment)
For example, considering escitalopram, if the response rate in the placebo group is 35%, and in the escitalopram group it is 55%, the Absolute Risk Reduction (ARR) can be calculated as follows:
ARR=0,55−0,35=0,20
-> NNT=1/ARR 1/0,20=5
This result indicates that, for every five patients treated with escitalopram, one patient experiences an additional clinical benefit compared to placebo. In other words, when five patients receive the treatment, only one of them shows a significant improvement that can be attributed to the drug itself.
The Balance Between NNT and NNH
While evaluating the benefits of a treatment, it is also important to consider the Number Needed to Harm (NNH) alongside the NNT.
NNH refers to the number of patients who need to be treated for one to experience an adverse effect attributable to the treatment. In clinical practice, a treatment is generally considered to have a favorable benefit–risk profile when the NNT is low and the NNH is high. Conversely, when both NNT and NNH are low, the treatment should be approached with caution. This comparison is particularly critical in the prescription of psychiatric medications.
In Conclusion
The Number Needed to Treat (NNT) is a quantitative parameter that expresses treatment efficacy numerically and serves as a valuable guide in clinical decision-making. However, this measure alone does not represent an absolute truth; it should be interpreted in conjunction with patient characteristics, clinical context, and alternative treatment options. Therefore, NNT is a practical and powerful tool that helps clinicians integrate scientific evidence with patient-specific circumstances.
